Atom Molecules and Ions
The molecules of an element are constituted by the same type of atoms. For example, a molecule of oxygen consists of two atoms of oxygen to form a diatomic molecule. In this section of Atoms , Molecules and Ions Atomic Mass Unit, Atomicity, Atoms, Chemical Equations, Empirical Formula, Formula of Binary Compounds, Gram Molecular Mass, Ions, Law of Conservation of Mass or Matter, Law of Constant or Definite Proportion, Laws of Chemical Combination, Molecular Formula, Moleculas Mass and Relative Molecular Mass, Percentage Composition, Relation Between Empirical and Molecular Formulae, Significance of the Mole, Symbols of Atoms and elements, The Mole Concept, Valency, Atoms Molecules Ions, , etc. have been explained. Watch the videos and master the concepts
Explore Concepts
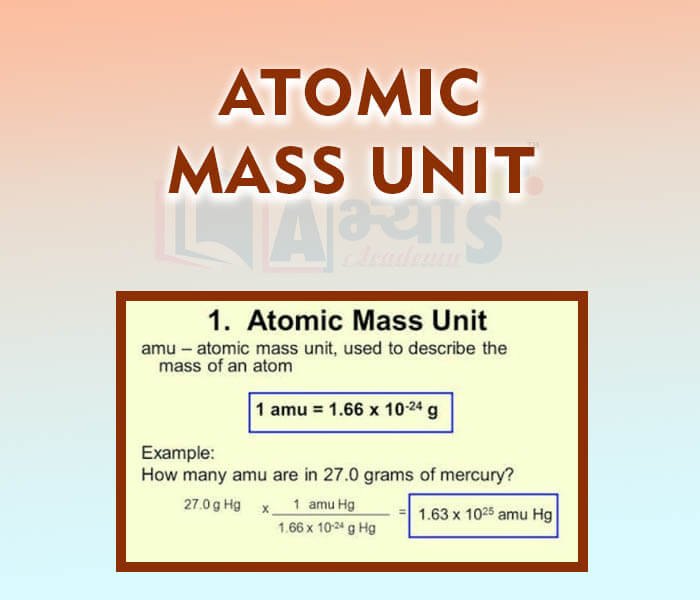
- Atomic Mass Unit
- Atomicity
- Atoms

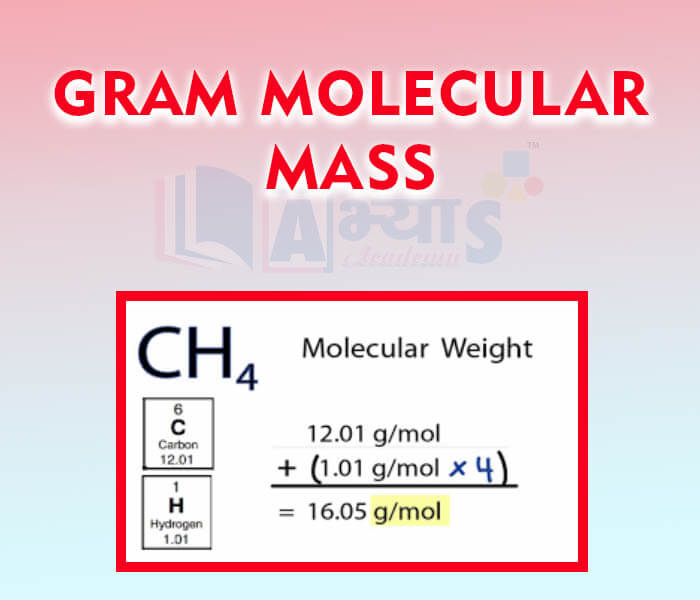
- Gram Molecular Mass
- Ions
- Law of Conservation of Mass or Matter

- Law of Constant or Definite Proportion

- The Mole Concept

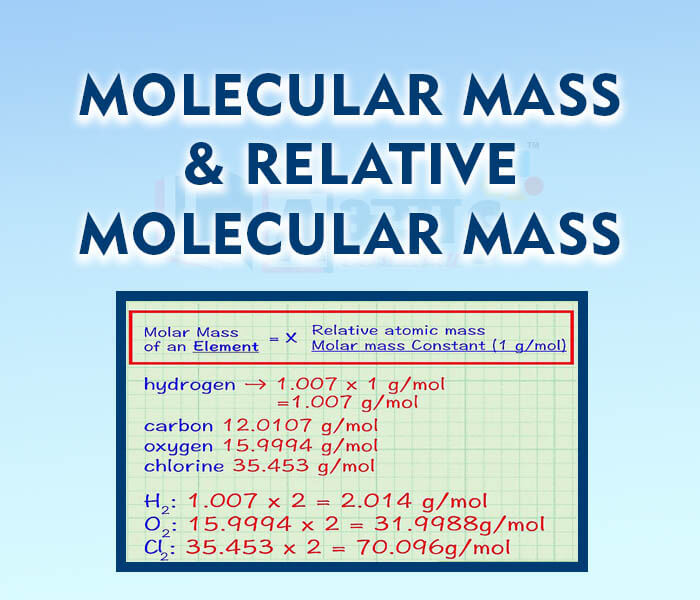
- Molecular Mass and Relative Molecular Mass
- Molecular Formula

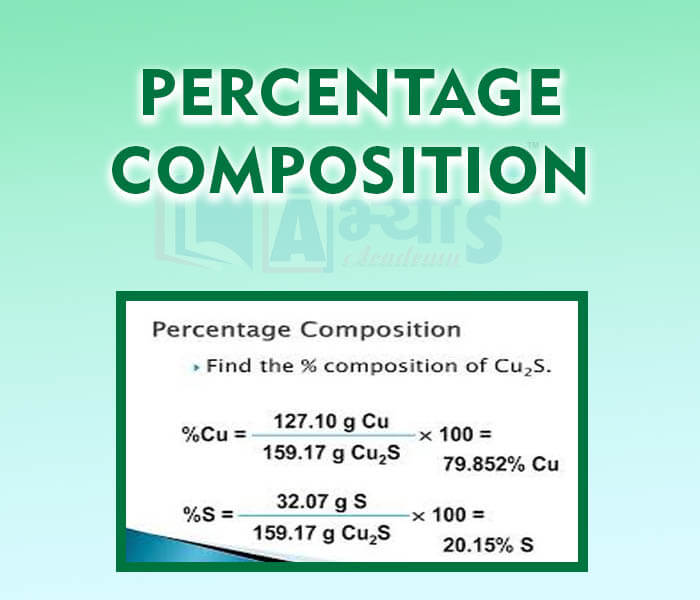
- Percentage Composition
- Empirical Formula

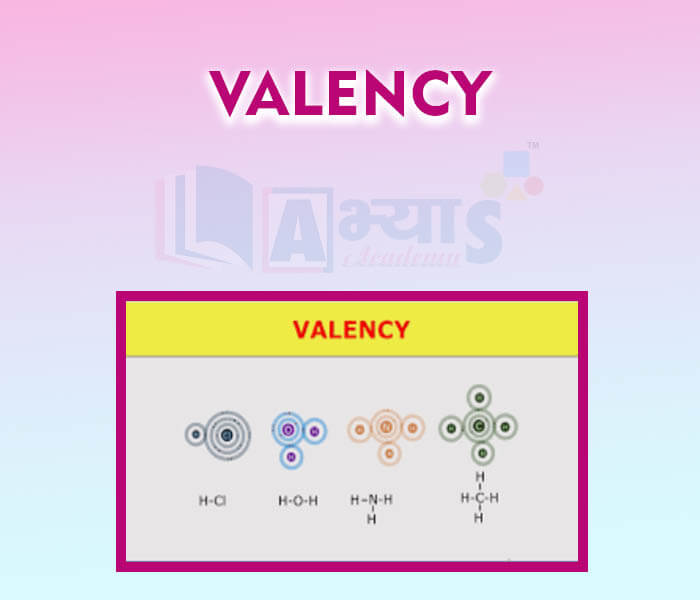
- Valency
- Formula of Binary Compounds

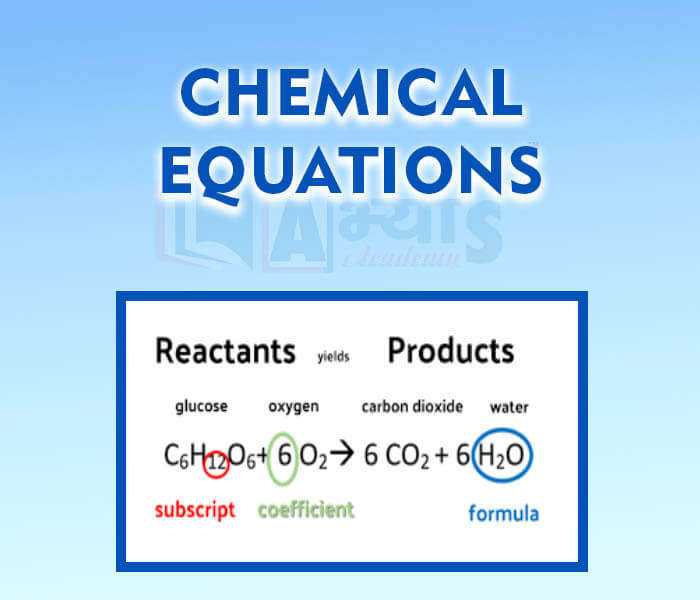
- Chemical Equations
- Laws of Chemical Combination

- Significance of the Mole

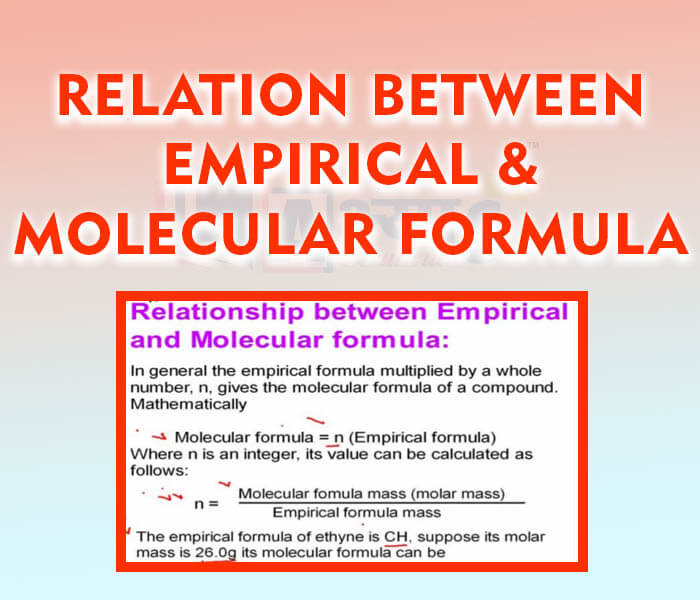
- Relation Between Empirical and Molecular Formulae
- Symbols of Atoms and elements
Students / Parents Reviews [10]
My experience was very good with Abhyas academy. I am studying here from 6th class and I am satisfied by its results in my life. I improved a lot here ahead of school syllabus.

Ayan Ghosh
8thOne of the best institutes to develope a child interest in studies.Provides SST and English knowledge also unlike other institutes. Teachers are co operative and friendly online tests andPPT develope practical knowledge also.

Aman Kumar Shrivastava
10thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thBeing a parent, I saw my daughter improvement in her studies by seeing a good result in all day to day compititive exam TMO, NSO, IEO etc and as well as studies. I have got a fruitful result from my daughter.

Prisha Gupta
8thAbout Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.

Shreya Shrivastava
8thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.






